Abstract
INTRODUCTION: Primary cutaneous peripheral T-cell lymphoma, not otherwise specified (pcPTCL-NOS) is defined as PTCL-NOS presenting only in the skin with no evidence of extracutaneous disease; this is a poorly-characterized diagnosis only recently recognized as a distinct entity by the World Health Organization-European Organization for Research and Treatment of Cancer (WHO-EORTC) classification system and the 5th edition of the WHO Classification of Lymphoid Neoplasms (Willemze et al., Blood 2019; Alaggio et al., Leukemia 2022). Few series exist and most include other histologies or patients with concomitant systemic disease. We aimed to characterize the clinical presentation and treatment of a large cohort of patients with pcPTCL-NOS.
METHODS: Through the Memorial Sloan Kettering Cancer Center (MSKCC) patient database, patients with a diagnosis of T-cell lymphoma and at least one visit at MSKCC between January 1, 2001 and December 31, 2021 were identified. Only patients with pathologically-confirmed PTCL-NOS confined to the skin at diagnosis were included. Patients with any WHO-recognized diagnosis other than PTCL-NOS were excluded, as were patients with concomitant systemic disease at diagnosis. All cases underwent pathology review at MSKCC. Patients were classified by the number of lesions and number of involved body regions at diagnosis (single lesion, regional [>1 lesion but only 1 body region], and multiple body regions). Management strategies were classified as systemic therapy, focal radiotherapy (RT), surgical excision, observation, combined modality therapy, or other approaches. Responses were assessed by reviewing clinical documentation. Progression-free (PFS) and overall survival (OS) probabilities were estimated by the Kaplan-Meier method and compared for various features using the log-rank test. PFS was measured from the date of initial treatment, and OS was measured from the time of initial diagnosis. For multiple variables of interest, a univariate analysis was performed to estimate associations with survival.
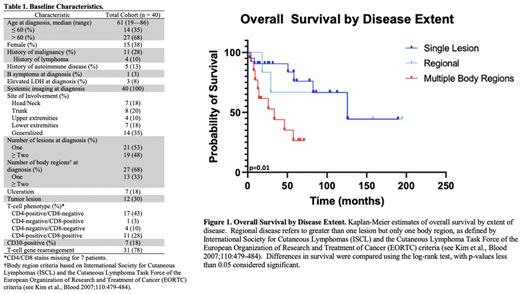
RESULTS: Forty patients with pathologically-confirmed pcPTCL-NOS were identified (Table 1). Site of involvement varied, as did disease extent at presentation (solitary lesion: 21/40; regional: 6/40; multiple body regions: 13/40). Classic B symptoms (1/40), ulceration (7/40), and tumor lesions (12/40) were uncommon. Initial treatment strategies varied by disease extent. For those with only a single lesion or regional disease, excision, focal RT, or excision followed by focal RT were the most common upfront strategies (21/27). Four patients with single/regional disease were observed. For those receiving upfront focal RT with or without excision, objective responses were achieved in 17 patients (ORR: 85%; CR: 80%). Among those who relapsed after upfront focal RT with or without excision (n=11/20), all relapses occurred outside the RT field. In patients with involvement of multiple body regions, systemic therapy with or without RT (6/13) was the most common approach.
Median PFS and OS of the entire cohort was 18.1 (95% CI 0.3-35.9) and 125.1 (18.7-231.8) months, respectively. However, by disease extent, median OS for those with a solitary lesion, regional disease, and involvement of multiple regions was 125.9, 125.2, and 33.6 months, respectively (p=0.01) (Figure 1). When analyzed by body region alone, patients with involvement of one versus greater than one body region at diagnosis had median OS of 125.0 versus 33.6 months, respectively (p=0.002). The presence of greater than one lesion (HR 7.6, 95% CI 2.5-22.9), disease across multiple body regions (HR 3.4, 95% CI 1.3-9.0), and tumor lesions (HR 10.1, 95% CI 3.3-31.3) conferred increased risk of progression. Similarly, disease across multiple body regions (HR 4.1, 95% CI 1.1-14.7) and tumor lesions (HR 9.5, 95% CI 3.0-31.0) conferred increased risk of death.
CONCLUSIONS: To our knowledge, this is the largest series of pcPTCL-NOS and importantly excludes all other WHO-recognized T-cell lymphoma diagnoses and those with systemic disease. Patients with a single lesion or regional disease had significantly greater survival than those with multiple regions involved despite relatively frequent relapses and differences in therapeutic strategy/intensity. Patients with multiple body regions involved underwent varied treatment strategies and experienced poor outcomes.
Disclosures
Ghione:Secura Bio: Consultancy; Kite Pharma: Research Funding; Kyowa Hakko Kirin: Consultancy; AstraZeneca Pharmaceuticals: Consultancy. Imber:GT Medical Technologies: Honoraria. Horwitz:C4: Research Funding; Crispr Therapeutics: Research Funding; Affimed: Research Funding; ADC Therapeutics: Research Funding; Daiichi Sankyo: Research Funding; Verastem/SecuraBio: Research Funding; Millennium /Takeda: Research Funding; Celgene: Research Funding; Kyowa Hakko Kirin: Research Funding; Seattle Genetics,: Research Funding; Yingli Pharma Limited and Tubulis: Honoraria; Takeda: Consultancy; Shoreline Biosciences, Inc.: Membership on an entity's Board of Directors or advisory committees; SecuraBio: Honoraria; ONO Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees; Kyowa Hakko Kirin: Consultancy; Daiichi Sankyo: Membership on an entity's Board of Directors or advisory committees; Cimieo Therapeutics: Honoraria; Affimed,: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal